
From School of Chemical Engineering
By Li Xiang, Song Ting
The research group from School of Chemical Engineering, Dalian University of Technology (DUT), has made a series of progresses in the integration of leukemia target discovery, anti-cancer drug development and biomarker identification, and has discovered a new therapeutic target for chronic myeloid leukemia (CML), developed an new anti-cancer drug lead for CML treatment, and revealed the BCR-ABL mutation-independent drug resistance to kinase inhibitors (TKIs). This series of researches integrates the chemistry strategy and the frontier technology of biology, focuses on the key scientific problems in the field of clinical medicine, and overcomes the problem of drug resistance in the field of leukemia treatment, which is a pioneering research in translational medicine and another important achievement of DUT in the intersection of medicine and industry.
Professor Zhang Zhichao’s research team has always been committed to developing Bcl-2-targeted anti-cancer drugs, and firstly discovered that Hsp70 protein can function as a Bcl-2 like protein and promote tumorigenesis in synergy with the classical Bcl-2 family in 2020. The research result has been published in one of the 82 top international journals included in Nature Index (J Bio Chem., 2020, 295, 12900). Based on this, the corresponding author of the study, Associate Professor Song Ting, proposed a hypothesis to integrate the multidisciplinary techniques of chemistry and biology to rapidly obtain the first specific Hsp70/Bim small molecule inhibitor S1g-2 from the library of Bcl-2 inhibitor molecules already created by the team as a new anti-cancer drug candidate. The related result was published by Associate Professor Wang Ziqian in Eur J Med Chem., 2021, 220, 113452.
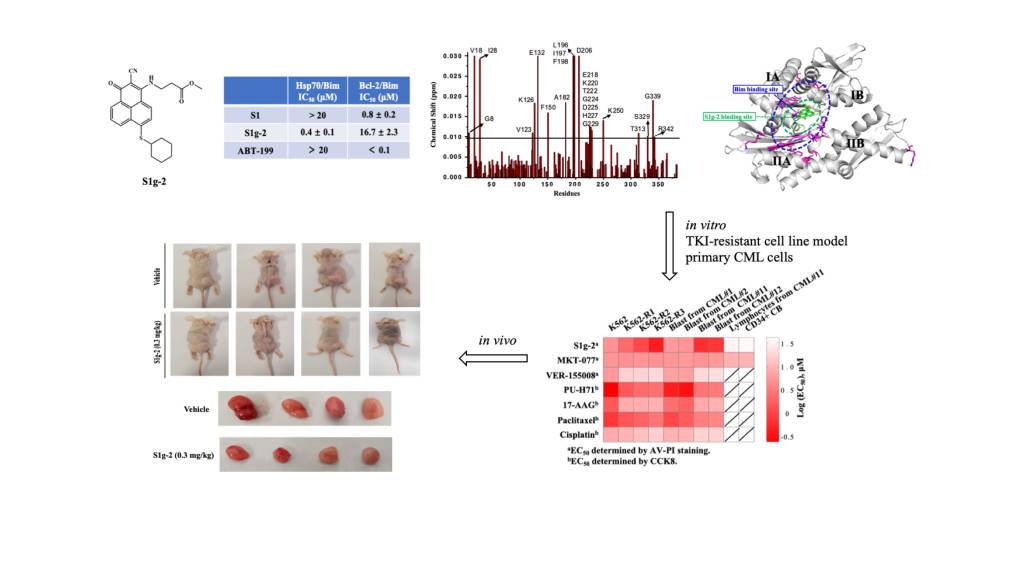
Then, the research group further developed translational medicine and innovative drug research for TKI-resistant leukemia. They screened a novel inhibitor of the Hsp70-Bim protein-protein interaction (PPI),S1g-2, from a Bcl-2 inhibitor library; this compound specifically disrupted the Hsp70-Bim PPI by direct binding to an unknown site adjacent to that of an allosteric Hsp70 inhibitor MKT-077, showing binding affinity in sub-μM concentration range. S1g-2 exhibited overall 5–10-fold higher apoptosis-inducing activity in CML cells, primary CML blasts, and BCR-ABL-transformed BaF3 cells than other cancer cells, normal lymphocytes, and BaF3 cells, illustrating Hsp70-Bim PPI driven by BCR-ABL protects CML through oncoclient proteins that enriched in three pathways: eIF2 signaling, the regulation of eIF4E and p70S6K signaling, and the mTOR signaling pathways. Moreover, S1g-2 progressively enhanced lethality along with the increase in BCR-ABL-independent TKI resistance in the K562 cell lines and is more effective in primary samples from BCR-ABL-independent TKI-resistant patients than those from TKI-sensitive patients. By comparing the underlying mechanisms of S1g-2, MKT077, and an ATP-competitive Hsp70 inhibitor VER-155008, the Hsp70-Bim PPI was identified to be a CML-specific target to protect from TKIs through the above three oncogenic signaling pathways. The in vivo activity against CML and low toxicity endows S1g-2 a first-in-class promising drug candidate for both TKI-sensitive and resistant CML. The result was published by Associate Professor Song Ting in Leukemia, the journal of Leukemia Society of America, which is ranked 3rd among hematology journals and 9th among oncology journals.
The study was supported by both the National Natural Science Foundation of China and the Dalian University of Technology-Liaoning Cancer Hospital Medical-Industrial Cooperation Program.
Link to the paper:
Editor: Li Xiang